It’s 2018, and if you need power storage, there’s a great range of battery options out there. You’ve got your bog standard flooded lead acid, AGM lead acid, lithium iron phosphate (LiFePO4), and of course the newer lithium ion chemistries (Tesla’s PowerWall being the most well known here).
And, if you’re building a system yourself, especially if it’s off grid, it’s incredibly likely that you’re using flooded lead acid, because they’re cheap, work well enough, and you can get them in most towns. They’re also quite reliable and can be quite long lived (15+ years) if maintained properly!

But: If you have an off grid lead acid battery system, it only stays cheap if you keep the batteries in good shape and don’t kill them in a few years - and a lot of off grid systems end up killing the batteries in a hurry. This either leads to an early battery pack replacement, or an awful lot of generator use - both of which are expensive, and generally at odds with the ideals of an off grid power system.
I’ve spent quite a while reading about lead acid from the view of, “How do they fail, and how do I maintain my battery bank to get the longest life out of it?” I’ve learned a lot, and also learned that it’s really hard to find good information on failure modes! I’ve dug through more than one PhD thesis to get information about some particular failure mode. This is a long post (over 11,000 words), with a lot of information, and I won’t apologize for that. I’ve tried to explain the concepts as I go into them, with a target audience of people who have large off grid battery banks. If you want something shorter, maybe check YouTube?
However, if you want to learn how to run a lead acid battery bank for maximum longevity (for your off grid power system or your RV), and to understand what’s going on in your battery bank, read on!
Lead Acid Batteries
Lead acid batteries. Toss some lead plates and some sulfuric acid in a case (ideally one that isn’t bothered by sulfuric acid), do a bit of a forming charge, and you’ve got a lead acid battery! Modern batteries are a good bit more complex (alloy structural grids with active material on them, various additives to the lead, and a much more complex forming process), but that’s it. Lead acid batteries are over 150 years old, and they’re still super common. Pretty much every vehicle on the road has at least one (including Teslas), they’re still the standard for backup power systems (data center UPSes, telecom equipment), plenty of golf carts and forklifts run on them, and the vast majority of off grid power systems on the planet store energy in lead acid batteries.
The basic chemistry is simple: You start with metallic lead on the negative side, lead oxide (PbO2) on the positive side, and an electrolyte of water and sulfuric acid in the middle (around a specific gravity of 1.28 or so). As the battery discharges, you end up with identical plates of lead sulfate (PbSO4) on both sides and very weak acid in the middle (lots more water in the mix and a specific gravity of around 1.05), plus an awful lot of electrons run out the terminals. Charge it up by reversing the current, and you get back (more or less) to the original state. Then add complexity in real world systems, because nothing is ever nearly so simple as the basic chemistry.
Lead acid batteries are still so popular because cheap, they don’t care about being cold (capacity is down and you can’t drain them fully or they freeze, but they’re not damaged by being cycled in the cold), they don’t mind the hot (it hurts lifespan, but that’s about it), they’re very safe (as long as they’re properly vented), and you can get them, in large quantities, on short notice. They’re “good enough” for an awful lot of uses - and, as I’ll talk about later, are getting a good bit better in the last decade!
That said, if you don’t take proper care of them (especially in an off grid system), they die quickly. A well maintained lead acid pack (with high quality batteries) can last well over a decade before it needs replacement, but if you abuse the batteries, the same pack will die (be murdered, really) in a year or two. What makes the difference? How you take care of them (and, to an extent, the system design - some off grid systems are improperly designed and will murder any battery pack you attach to them). Put your acid resistant gloves on and let’s dive in!
Flooded/Gel/AGM
There are three main categories of lead acid batteries on the market: Flooded (FLA), Absorbed Glass Mat (AGM), and gel. They all use lead plates and sulfuric acid, but the nature of how the lead and acid interact differs rather dramatically between the three types.
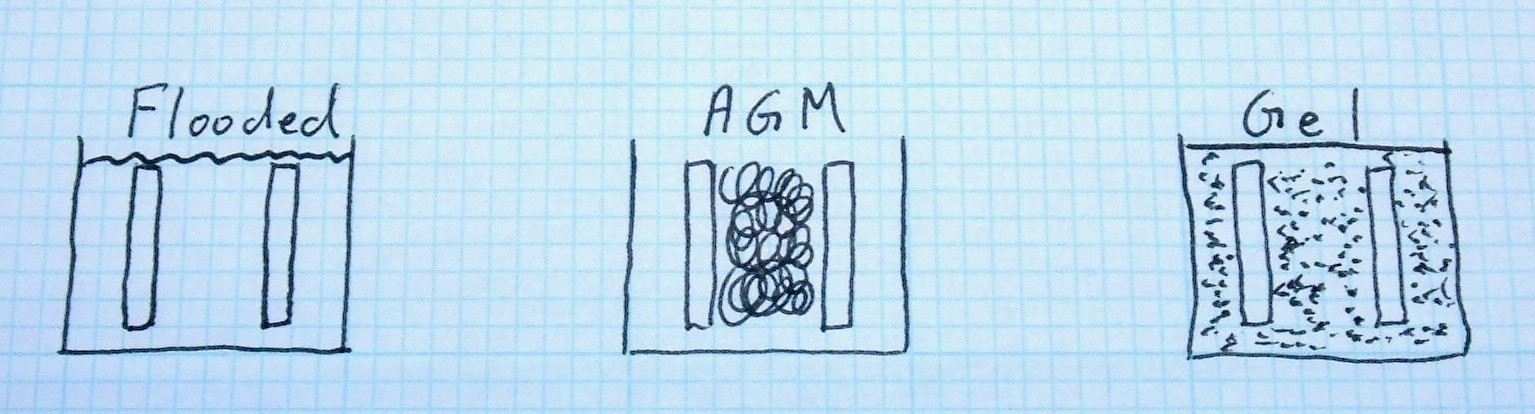
Flooded lead acid are what most people think of when they think of lead acid - a bunch of lead plates hanging down into a bath of liquid sulfuric acid and water that’s free to slosh around. The acid can move freely around (and into) the plates, it bubbles when driven into gassing voltages, and you can pop the top to examine the specific gravity of the electrolyte and add more water. Most car batteries are this type, and a lot of deep cycle batteries are of this design as well. Some of them are “sealed” and “maintenance-free,” but if you pop the tops, you can see the acid sloshing around and add water if needed. Mostly those terms just mean, “We think there’s enough acid in there to last the expected life of the battery and by the time it’s a problem, it’s out of warranty.” Don’t think it means that there are catalytic recombiners at the top or anything like that.
AGM refers to “Absorbed Glass Mat” batteries, and is a type of VRLA (Valve Regulated Lead Acid) battery. In this type of battery, there are woven mats of glass fibers between the lead plates, and they’re soaked in acid - but not fully saturated. This is, by far, the most common type of sealed lead acid battery. Since the acid is absorbed in the glass mats (as implied by the name), they don’t leak. There’s no liquid acid sloshing around in the battery, so you can install them in almost any orientation (helpful for motorcycles). They’re “maintenance free” - but also “maintenance impossible.” You can’t check the specific gravity, can’t add water, and when they fail for whatever reason, you recycle them.
Gel is another type of VRLA battery, and involves the acid being a thick gel instead of a liquid - usually silica is added to thicken the acid. They’re reasonably rare outside certain circles, and are a special type of touchy about voltages, so you want nothing to do with them for an off grid power system. Most “lead silicone” or “lead crystal” batteries are this type, and are mostly marketing hype, as far as I can tell. This type relies on cracks in the hardened acid to let gasses equalize between plates, and they work, but they’re not very well suited to off grid or RV use.
What’s this “valve regulated” thing about? For both AGM and gel batteries, they’re fully sealed batteries. Normally, during charging, hydrogen and oxygen are created from by splitting water in the electrolyte while up at the absorb voltage. A flooded battery normally just vents this to the air, so you have to add distilled water on occasion (pure water can accurately be called hydrogen ash - it’s just oxidized hydrogen). VRLA types try to recombine this internally - the oxygen and hydrogen get back together inside the battery, and go back into the electrolyte as water. The valve comes into play when this doesn’t work - if the charge rate is too fast or the voltage too high, the hydrogen and oxygen can’t recombine fast enough, the pressure builds up, and the vent lets the gasses out so the casing doesn’t explode. This loss is permanent. You can’t add new water to this type of cell, so the venting is a permanent thing that reduces the longevity of the cell. It’s a safety measure (it does beat a cell exploding from overpressure), but this is not something that should be happening in normal operation.
Proper Uses of FLA and AGM
Opinions vary on the proper use of flooded lead acid versus AGM - but I’ll offer mine here. For a daily cycled off grid system (or an RV that’s lived in for long periods of time, which amounts to the same thing), you really, really want a flooded battery. Flooded lead acid is the most robust variety of lead acid - it tolerates abuse that AGM won’t, you can accurately check the health (through specific gravity measurements), and you can add water when it’s low. For a daily cycled pack, this is what you want. Flooded packs take some useful types of abuse quite well (overcharging, which is far better than undercharging). The key benefit of flooded is the ability to check the specific gravity and add water, though. Specific gravity of the electrolyte gives you “ground truth” about the state of charge and battery health. The alternative is voltage, but that usually requires letting the battery pack sit with no loads at all on it for a few hours, and that’s annoying in an off grid system (you’re without power while you let the pack sit idle). Plus, it’s far less accurate than specific gravity.
AGM is amazingly well suited to standby use - almost every UPS (uninterruptable power supply) battery out there is AGM - the batteries live on a float charge and are cycled a few times in their life (a few dozen, max). It’s also well suited to infrequent cycle use, assuming you get a deep cycle AGM (used UPS AGMs aren’t worth it even if you’re given them for free - this is a trick to avoid having to pay disposal fees, and there are usually enough suckers out there that it works well). For a remote cabin that’s used on the weekends during the summer, or an RV that’s used a month or two a year, AGM is a great battery (assuming you keep it on a good charger when not in use). AGM batteries have a finite lifespan - the compromises required to keep them from gassing too much mean that you’re dealing with perpetual positive plate corrosion, but if you want a battery you can install, configure voltages, and ignore for 5 years, AGM is the way to go. They tend to have a lower self discharge rate than FLA (especially in the cold), and they tend to be better at providing a lot of current with less voltage sag. The downside is that you really can’t maintain them at all - there’s no way to check specific gravity, and if you do vent them, well, such is life. You can’t add water. No, you shouldn’t break the vents to add water…
Another reason you might want AGM is if you’re building a battery pack that can’t be guaranteed to remain within a certain set of angles. For boats that are regularly offshore, AGM is the way to go because the pack won’t spill in heavy seas. If you do serious off road work with an RV, this is worth considering as well.
The remainder of this post is mostly focused on flooded lead acid in daily cycle use. That’s what an off grid power system is likely to have, and that’s what an RV is likely to have (if used regularly). If you’ve got an AGM pack, some of this will apply, and I’ll mention it when it does. If you have a gel pack, you probably got suckered by slick marketing, so… good luck!
Sulfation
Ah, sulfation. The common term for every lead acid battery failure. You can buy desulfators from any number of legitimate and sketchy looking sources. If your battery fails, someone is almost certain to mention sulfation while nodding knowingly. And, to be fair, it happens literally every single time you discharge your battery - but reverses every time you charge it.
Sulfation is the conversion of lead dioxide and elemental lead (the positive and negative plates, respectively) into lead sulfate (extracting sulfur from the electrolyte, which makes it more and more dilute as you discharge the battery). Charging the battery reverses the process, increasing the sulfuric acid concentration and reverting the plates to lead and lead sulfate. The sulfation from cycling is usually called “soft sulfation,” because the crystals are small, soft, and easily reversed.
Generally, when people refer to sulfation as the cause of death of a battery, they’re referring to hard sulfation, or crystalized sulfation. This happens from chronic undercharging, when the cycle sulfation hardens and turns into insoluble sulfates throughout the plates. These clumps can interfere with acid flow into the active lead compounds, and they can expand and physically break the plates apart. This is all sorts of bad, and it leads to a battery that can’t hold a charge and can’t provide anywhere near rated current. It also looks charged earlier than it should, because the voltage rises (it can’t accept a charge, so it looks full when most of the plates are still covered in crystals and the specific gravity is very low). From the perspective of the charger, it’s done, but a specific gravity test will show lower than specified values for a fully charged battery.
Fortunately, the formation of hard sulfation is pretty well understood - undercharged batteries do this. A lead acid battery has to be fully charged, quite often, or it will suffer hard sulfation and lose capacity. Lithium batteries are happiest at a middle state of charge, but lead is happiest fully charged - and, if you don’t fully charge it regularly, lead will die on you in a hurry. If you don’t have enough panel capacity to ever fully charge your bank (or to only charge it fully in direct sunlight), you’ll kill the batteries in a year or less unless you compensate with generator use.
What’s fully charged? If you have a flooded battery, check the temperature compensated specific gravity. That will tell you (per your manufacturer’s datasheet) if your cells are fully charged. You should really be checking them somewhat often, at least early in the system lifespan. If you have an AGM cell, well, good luck. Temperature compensated charging voltages and charge termination current are the best you can do.
What’s that? You don’t have a manufacturer’s datasheet and can’t find one? Well, you probably don’t have a particularly good battery for off grid use, then. Good luck! Measure the specific gravity when the cells first go into service (after a hard equalization charge), and use that as a reference.
Positive Plate Corrosion
If undercharging a battery will kill it in short order, what will overcharging do? Two things - gas the battery, and corrode the positive plate. Positive plate corrosion is the other common way to kill a lead acid battery - the plate eventually corrodes enough that you lose surface area, volume, and the ability to hold a charge. The positive plate is also often found at the bottom of the battery case in fragments (as it loses pieces, they settle to the bottom).
So, why would you want to do this? Simple: Unless you’re massively abusing a battery, this will take much closer to a decade to kill the battery. Sulfation will kill it in under a year. You have to pick the side you want to run on, and the corrosion side of the knife is a far better place to run than sulfation.
Short of having perfect specific gravity monitoring of every cell (which will probably drive you nuts in short order from the variance), you can pick which side of the “knife edge” to run your battery on - sulfation or corrosion. Corrosion will take radically longer to kill the battery, so that’s where you want to run. Mildly overcharging a lead acid battery is far, far superior to mildly undercharging - at least if you have a flooded batter you can water.
If you have an AGM battery, don’t overcharge it too much or you’ll gas it more than the recombiners can handle, vent electrolyte, and permanently damage it. Even if you don’t vent, AGMs run a lot hotter during charging from the recombination cycle, and you can get one a lot hotter than it should be with sustained charging (this is far less likely off grid, but it’s something to be aware of). So, again, temperature compensated charging is the best you can get with AGM. But, you still need to fully charge it or you’ll sulfate the plates up badly. If you’re getting the idea I’m not a huge fan of AGM for a daily cycled system, you’d be right…
You can also get very, very rapid corrosion (on both plates) by exposing the plates to air. Keep your batteries watered properly!
Active Material Shedding
Both sulfation and corrosion can break material away from the plates. And, as batteries age, material slowly breaks away as well. There’s not much you can do about this, beyond running the batteries as advised in the datasheet.
If you get enough lead debris at the bottom, this can actually short out plates - this is a fatal failure. Fortunately, modern deep cycle batteries leave space for this, and often have wrappers that help keep shed material against the plates, where it can’t cause problems.
I mention this mostly as an argument against snake oil “Take a 10 year old battery and make it brand new again!” type products (promotors of these attempt to spam my blog quite regularly with their nonsense). If 90% the active material is sitting on the bottom of the battery case as corrosion products, how, exactly, are they going to get it back in place with their patented fizzies or patented zapping cycles?
Acid Stratification
Stratification is a quirk of lead acid that’s only found in stationary flooded batteries. The vast majority of lead acid batteries (in vehicles) are vigorously sloshed around regularly, so the acid is always well mixed. And AGM batteries don’t have this problem as the acid can’t move around to start with.
Charged electrolyte is “more acid” than discharged electrolyte. And “more acid,” in the case of the acid in our lead batteries, means “heavier.” Given enough time, the heavy acid can settle to the bottom of the battery, while lighter water comes to the top. This is really, really bad for longevity - the bottom of the plates is being attacked by a very strong acid, and the top of the plates can barely contribute to energy output because there’s nothing to work with.
Stratification is mostly an issue with underpowered charging systems. If you don’t have enough power to fully charge the battery bank regularly, and can’t get enough charging amps in to gas the batteries regularly (an under-paneled setup), you’re much more likely to run into this issue. The main symptom of this will be low specific gravity electrolyte when the cells should be fully charged (though sulfation also will show the same readings).
The good news is that it’s easy enough to solve - just gas the batteries for a while. The bubbling helps mix the electrolyte and even things out throughout the cells. This may require generator use, but you should see the specific gravity rise as the acid evens out around the cells.
Preventing stratification is another advantage of running on the overcharge side of the knife. You’ll gas the cells more often and keep things mixed.
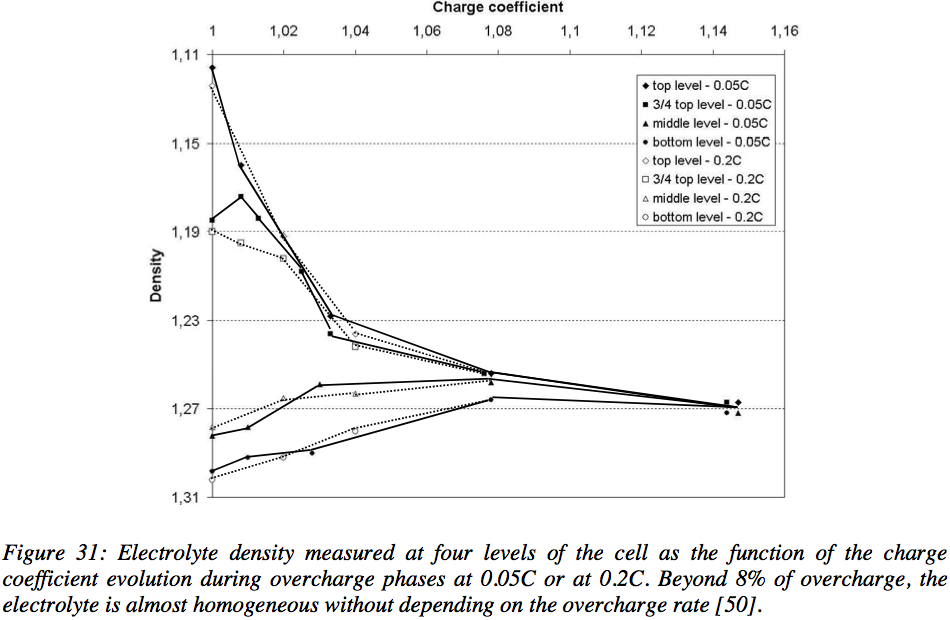
NGUYEN Thi Minh Phuong’s thesis (an amazing read if you care about lead acid batteries) includes this helpful chart - regardless of the charge rate, if you can cram an extra 8-10% of charge into the battery bank, stratification pretty much goes away as a concern.
Lazy Batteries
One other quirk you may see in an off grid system is the “coup de fouet” effect - a more pronounced voltage dip on the start of discharge than expected. If you keep the battery under a constant current discharge, the voltage will then rise back up in a few minutes to where you expect it to be, and will remain there during the rest of the discharge. If you have a conservatively set low voltage cutoff and a small bank, this dip (with a heavy load) may be enough to trip low voltage cutoff, even though the battery bank has lots of capacity left.
You may also see the inverse effect if you’ve been running at partial state of charge for a while, with no sun, and you start charging from the generator at a good rate (my generator/charger setup will charge around C/13 - I’d prefer higher, but I’ll take what I can get). The voltage will rise up under charge, but with a steady charging current, the voltage will then dip back within a few minutes before starting to rise as the battery pack charges. Study of the “coup de fouet” of lead-acid cells as a function of their state-of-charge and state-of-health has a good graph of this behavior.
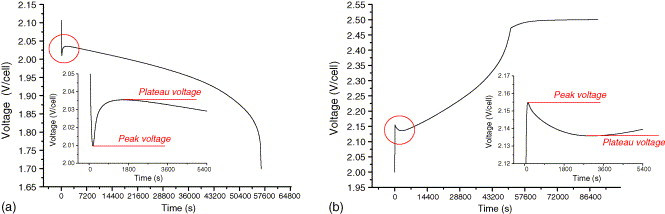
This is a normal enough behavior of lead acid batteries, and it relates (as I understand it) to “getting things flowing” in terms of the electrolyte flow into the plates and getting rid of any surface corrosion interfering with the charging or discharging. Basically, the battery has gotten lazy from sitting around not doing much, and it takes a while for it to warm up and start putting out current (or accepting a charge). It’s not sulfation, it’s not a worn out battery bank. It’s just something lead acid batteries do. Ride through the dip and the voltage will go back up (or come back down).
But if you’re only using voltage as a state of charge indicator, this can catch you by surprise.
Thermal Runaway (AGMs)
Thermal runaway of batteries is something mostly seen in the lithium ion space, but it can happen to lead acid batteries under charge as well. What happens is that as the battery gets hotter, the internal resistance drops - so the battery will accept more current at a given voltage. This extra charging current causes more gas generation, and as the gas recombines back into water, heat is released. This can turn into a positive feedback loop, rapidly heating the battery beyond service limits. As the battery generates more and more gas, some of this is likely to vent, improving the gas flow between positive and negative plates, and increasing the heating. This does not end well, and even if you catch it, it causes permanent capacity loss in cells. If you don’t catch it, it can often end with blazing hot battery components and sulfuric acid scattered around your battery room when the battery finally explodes.
C&D Technologies has a great whitepaper on runaway in AGM batteries, if you want to learn more.
The good news is that flooded batteries aren’t nearly as prone to this (they vent their gasses instead of recombining them so heat generation is significantly less), and off grid power systems don’t generally produce enough power for this to be an issue. An off grid system won’t keep a battery in float indefinitely either. Temperature compensated charging should mostly prevent the issue - as the pack temperature rises, the charge voltage will drop. If you have a large pack and only one cell enters runaway, it could still cause you some excitement, but this isn’t a common failure method for off grid power systems.
How Then Should We Charge?
Fully, Properly, and Regularly! That’s the secret to long lead acid battery life. However, actually doing that is a bit complex, so I’ll dive into it more. But if you want your lead acid batteries to live long lives, you’ll have to do a bit of work, starting with the system design. And you’ll need to avoid “killing them with kindness.” Trying to be nice to lead acid batteries, in an off grid system, will utterly destroy them in very short order.
On Grid vs Off Grid Charging
Up until fairly recently, all the lead acid charging advice and voltages implicitly assumed grid tied charging - deep cycle flooded lead acid batteries were used for things like golf carts, forklifts, and floor cleaners. UPS systems sit at a float charge when they’re not in use. And in all of these situations, “eventually charged” is good enough. There’s an infinitely stiff voltage supply (the power grid and attached charger) that can charge the batteries, so as long as they charge fully by the next shift (often a 16 hour charge cycle or longer), everything works and the batteries live out a long and happy life. You can get away with charging at or slightly above float voltage, if you have long enough, and this is a wonderfully gentle way to charge lead acid for long life.

Off grid is a totally different beast. Most off grid systems are charged by solar or wind - hardly the most reliable forms of energy. If you’re incredibly lucky, you might have a small micro-hydro setup that gives steady base load year round (or, at least, in the winter), but that’s pretty rare. And, worse, for a solar based setup, you only have limited hours of sun in the day before you’re finished charging - regardless of the state of charge of the cells!
If your system never fully charges your batteries (or can’t get enough amps in to gas them properly), you’re going to have a bad time with lead - they’ll sulfate up, stratify, and generally not come remotely close to lasting as long as they should.
So, let’s dive into how you should set up a lead acid bank for optimum life off grid!
Cell Layout
This is subtle, but important. Try to avoid paralleling lead acid batteries together. You can sometimes get away with two strings in parallel, three is doable if you’re really, really careful, and beyond that? Forget it. You’ll just murder all the batteries in short order. A single series string is, by far, the best option for cell layout, and you should go out of your way to set your system up as a single series string.
Lead acid charging is a messy, handwavy affair. The cells that fill up first will sit and gas, while the lower state of charge cells catch up. As the cells age, internal resistance changes, and this doesn’t always happen at the same rate for different cells. For cells in series, things more or less work - the current is forced through all the cells, so they’ll all eventually charge (as long as they’re not shorted out internally). You may go through a lot of water, but you can get all the cells to charge fully, even if the pack is quite worn out.
With cell banks in parallel, it gets a lot harder to charge properly. Lead acid internal resistance is heavily temperature dependent (this is why cold battery banks sag so badly, and why temperature compensation is so vital). If you have a bunch of strings in parallel, it’s likely that one of them will be warmer than the rest - which will lead to lower internal resistance, higher current through that string, and overcharging the warmer string while undercharging the colder string.
And there are a lot of other factors that can lead to the same result.
The takeaway? Try not to parallel your batteries. If you must, be careful about equal length cables and trying to keep the batteries all at the same temperature. But, really, a single series string is going to make your life a lot easier.
How do you do a really, really high capacity series string? With really, really big cells! Beyond a certain capacity, you’re not buying batteries - you’re buying cells (so 2V nominal for lead acid). Buy the amp-hour capacity you need, and string them together in series.
The largest Trojan solar energy cell is the SIND 02 2450. This is a 2V cell rated at 2540Ah (C/100 rate) and 4.9kWh per cell. A 48V string of these (24 cells in series) is a whopping 117kWh pack. That’s big enough for even a quite large off grid home!
Temperature Compensation
If you want long life out of your batteries, and they don’t sit inside at a well controlled temperature, you must have battery pack temperature sensing and temperature compensated charging. This is not optional. Also, because some cheap controllers will do it based on the temperature of the controller, it’s important that the temperature in question be the temperature of the batteries. Ideally, your controller will come with a nice remote temperature probe you stick on a battery in the center of your pack (any good charge controller will support this).

What is temperature compensation, and why does it matter? The short answer is that it pushes the charge voltages up when the battery bank is cold, and reduces them when the bank is warm, and this is vital to keep the batteries healthy.
In more detail, the internal resistance of lead acid (and most other chemistries) is dependent on temperature. When the battery is warm, the battery works more efficiently (the reactions are faster) and there’s less voltage change for a given current (both on discharge and charge - you’ll notice that cold lead acid is really saggy in terms of voltage). When charging, and the battery bank is cold, you need a higher voltage to cram enough current through the plates to properly charge things. Conversely, when the battery bank is really hot, you don’t need as much voltage to put the proper amount of charging current through the plates.
Your battery data sheet should have the temperature compensation coefficient, but if you can’t find it, -0.005V/(cell * degree Celsius) should do it, and 25C is a normal reference temperature. You can do the math in F if you’d like, and it works out the same. Note that this is per cell. So, for my 48V office bank, I have 24 cells. At -10F (-23.3C), that works out to an extra 5.8V on my bank to properly charge it - if I didn’t have that, the bank would be chronically undercharged all winter, and that leads to sulfation and early battery death. Don’t go there. Though, actually, my battery bank never gets quite that cold. There’s an awful lot of mass, and I rarely see below about -5C on my batteries. And my battery bank is sort of a worst case situation, as it’s sitting outside my office in an entirely uninsulated ~deck storage container~ totally legitimate battery box.
Battery Temperature
One of the significant advantages of lead acid over lithium is that the chemistry just doesn’t care that much about temperature. Over the vast majority of temperatures found on the planet (or, at least, where you’re likely to find humans), lead acid batteries work just fine.
If you don’t have a good way to regulate battery temperature, then don’t worry about it. There’s nothing you can do about the pack temperature, and as long as you take care of the cells, it will last you a long while.
However, if you do have a way to regulate battery temperature, consider the following:
Above freezing, the colder the battery is, the longer it will last. You get less energy out of the pack when it’s cold, but in the summer, with plenty of solar energy coming in, this is rarely an issue. For every 10C above the rated 25C, the battery life halves. However, for every 10C below, you get a corresponding increase in battery life. Spending some energy cooling batteries in the summer may pay off nicely in lifespan!
Below freezing, the freezing point of a lead acid battery depends on the state of charge. Fully charged, lead acid freezes around -90F. Fully discharged, it will freeze around 20F. At a 40% state of charge, it will freeze around +5F. So don’t drain it fully in the winter if it’s brutally cold.
To make your life easier, lead acid batteries are really heavy. They have a ton of thermal inertia, and if you keep them out of the wind, they don’t change temperature quickly. Plus, when they’re charging, they get warmer. Despite not having any thermal control on my office pack, the pack just doesn’t swing that rapidly in temperature. I rarely see my pack much below freezing in the winter, and even on hot summer days, it doesn’t get anywhere near ambient temperature in the afternoon (I may see 30C/86F cell temperatures on 40C/104F afternoons, but that’s pretty rare).
Specific Gravity & Voltage Testing
Another advantage of flooded lead acid (as opposed to sealed lead acid) is that you can easily check the specific gravity of the electrolyte. This is the ground truth about battery health. Voltages, especially on a continually loaded system, are not perfect for telling state of charge and health, but the specific gravity of the electrolyte, temperature corrected and referenced against the datasheet, will tell you exactly how a cell is doing.
There are two major options for measuring specific gravity: Temperature compensated hydrometers, and refractometers.
You don’t want one of the old antifreeze testers - those aren’t nearly precise enough for lead acid battery maintenance.
What you want is something closer to this: A temperature compensated unit designed for battery monitoring. This is my current favorite unit for quick testing - suck acid in while making sure there are no bubbles, and read the values.

A refractometer is a much higher precision instrument, and I use this somewhat regularly for higher precision readings. They’re not the cheapest thing on the planet, and you are having to drip acid around outside the cells, but the precision is worth it. Also, the thermal mass of a refractometer pretty much solves temperature compensation for the measurements - just be sure your refractometer is at the target temperature (plus or minus a bit), and the few drops of acid will take the temperature of the tool. Awesome!

This is an interesting little gizmo to use, because you peer through it to see the waterline on the display (I’ve got some water in, because it was cold and I didn’t want to go outside to suck battery electrolyte). It’s quite precise, as long as you’re doing the temperature compensation, but it’s certainly less convenient than the battery tester above. However, this also will test various coolants and washer fluid as well (if you’re the type who still does that instead of just using the 50/50 premix).

What’s most important is the temperature compensated specific gravity at the end of charge. If it’s not high enough, the cells aren’t fully charged - that’s the way to sulfation. You can either measure every cell, or take a random sampling somewhat regularly. I tend towards the second, though the first is certainly more robust. It just takes longer. I’ll do a random sampling of a few cells in the afternoon after charging, and if they’re all up at the fully charged SG, I don’t bother taking more readings.
You should also check the voltage of your batteries (or cells) somewhat often. Again, fully charged is ideal, but you don’t want to be under active charging. Turn off the charger and try for a consistent load while you measure. You’re looking for more or less equal voltages between the cells or batteries (within some tolerance - they’re never going to be exactly equal and you’ll drive yourself nuts trying to accomplish that).
Voltage as a state of charge indication is mostly useless for off grid power. It will get you in the ballpark, but voltage is only useful after the batteries have been sitting, with no load, for a few hours. That never happens off grid, so it’s better to rely on other methods for estimating state of charge.
If the fully charged specific gravity or the fully charged voltages vary significantly, it’s time for…
Equalization
Equalizing is often talked about as some magical process that solves all known issues with lead acid batteries. It’s not nearly that complicated. Equalizing is the fine art of beating the crap out of the cells with an excessively high charging voltage to force current through already-charged plates (and gas the cells). This accomplishes several things, most of which tend to increase the measured specific gravity at the top of the electrolyte by solving various issues in the cell.
If the cells do have some hard sulfation building up on the plates (these crystals hold what should go back into the electrolyte, so the specific gravity will be lower), the higher voltage and agitation is better at breaking up these deposits and letting them dissolve back into the electrolyte. It can’t solve everything, but it will often help with the early stages of the deposits.
Equalize voltages also gas the cells - hard. A lead acid bank while equalizing is a very noisy bank of batteries from all the gas bubbles. This serves to vigorously mix the electrolyte (stratification is another reason the electrolyte at the top of the bank can be weaker than desired), and should resolve stratification issues - at least for a while.
However, this aggressive overcharge causes rapid corrosion on the positive plates and it uses a lot of water - it’s really hard on the cells. So, there’s no point in doing it unless the batteries actually need it. If you’ve got charging voltages set correctly, and enough panel/wind/hydro/generator for your bank, you may never need to equalize your bank.
Some manufacturers suggest equalizing every month or two. Some suggest every 6 months. And some suggest only doing it if the cells show they need it. Trojan is in the last category (at least on some pages - they’re pretty inconsistent across the website), and I think this is really good advice. Their advice is to only equalize a cell if you see widely ranging specific gravities (>0.030 min/max spread across the bank), or widely ranging voltages between batteries (0.30V for 12V batteries, 0.15V for 6V batteries - so 0.05V/cell difference). If the cells are closer than this, there’s no good reason to equalize.
Interestingly, Trojan also recommends that solar systems equalize every month, for 2-4 hours. I expect this is based on the older charging voltages, because they’ve changed the off-grid charging voltages recently!
Before you equalize a pack, charge it fully and top the water off (in that order - I’ll talk about this more later). You’ll go through a lot of water with a few hours of gassing it, and exposing plates is a sure way to kill batteries. Also, keep an eye on temperature - pack temperature will head up in a hurry. I don’t let my pack run past 40C/104F when equalizing. The actual limits are a bit higher, but it’s a good idea to pick a cooler day to equalize. However, if you’re dealing with hard sulfation, hotter temperatures may help break it up a bit better.
Let the pack bubble until the specific gravities stop rising. You should check water levels when you finish and add water again if needed. Your manufacturer will have more specific directions.
Some manufacturers suggest a periodic equalize cycle for AGMs, some say “Never equalize AGMs.” Check with your manufacturer. If you equalize AGMs, you may drive the internal pressure high enough to cause the relief valve to let out some of the gas, and that’s electrolyte you never get back.
Charging: Bulk, Absorb, Float
You’ll see some potentially unfamiliar terms tossed around in the context of charging lead acid batteries, but they’re really quite straightforward. This is a charge diagram from my battery bank on a day when I got a clean charge curve. Starting from the left side, the sun comes up around 0830. From there as the voltage and charge current rise, the battery is in the “Bulk” phase of charging. At about 1030, when the voltage peaks out and the charge current starts dropping, the battery is in the “Absorb” phase. And, right before 1600, the system terminates the absorb phase and switches to the “Float” phase for the remainder of the sunlight, which goes down a bit after 1700. This chart represents a winter day of sun after a few days of no sun.
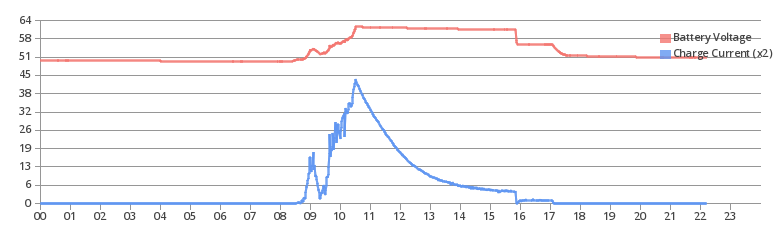
“Bulk” charging (or “constant current” in the context of grid charging) simply refers to the point at which the battery is below the absorb voltage, and the charger is pumping in as much current as it can, at whatever voltage the battery bank is. A grid tied charger will typically have a set charge current, whereas an off grid system will charge with whatever is available (you can see the charge current rise as the sun gets higher in the sky, and you can see the impact of early morning clouds on the charge current). Charging here is fairly efficient for lead acid. As the battery charges, the voltage will gradually rise - you can see in the chart above that the current is whatever is available, and the voltage rises proportionally.
Once the voltage rises up to the temperature compensated absorb voltage, the bank enters the “absorb” stage of charging. In this stage (usually from 80-85% to 100%, though may start higher if you don’t have as much charge current available), the charger holds the voltage constant, and the charge current tapers off as the battery bank fills. For a small off grid system, especially one that doesn’t have enough panel, you’ll likely remain in this state all afternoon trying to charge the battery bank. Absorb can take several hours to complete, and when dealing with a bank that’s been running at partial state of charge for a week, it may take a few days to catch everything up. On my chart, you can see the voltage gradually dropping during the absorb phase - this is temperature compensation in work. The battery peak heats up significantly during absorb (from 4C to 12C on this day), and the voltage drops as the pack heats up.
However, if you have enough panel, or the bank isn’t very empty, the absorb stage can end before the sun goes down (as it did here). Absorb ending can be determined by specific gravity, or by the charge current into the bank. When it ends, you’ve got a few options. You can turn the charger off entirely (and run on batteries, despite there being good solar), you can stay up at absorb voltage (gassing the batteries, going through water, and corroding the positive plate), or you can drop down to float voltage (which is what you should do).
“Float” voltage is a lower voltage than absorb. This can be thought of as “neutral” for batteries. The bank is charging very slowly (mostly countering self discharge), and the charge controller will provide enough power from the panels to hold the voltage there. This means, practically, that your loads will be running directly off solar without the battery bank participating (though it will still help out for sudden demand spikes or if a cloud shades the panels). A bank can sit here indefinitely (and telecom/UPS batteries do), but an off grid system tends to run out of sun sometime in the evening and start discharging the bank.
There’s no such concept as a “bulk voltage” - the bulk phase simply refers to the “cram in as much current as you can” phase.
Occasionally you might see a reference to a “finishing charge” - this is generally a final quick voltage spike after charging at a lower voltage to make sure the battery is fully charged, but it’s not a concept that applies for off grid use (that I’ve seen). I suppose you could use it, if your charger supports it, but I’ve not seen one that does.
“Three stage” charging refers to the bulk, absorb, float cycle. “Two stage” or “CC/CV” charging (constant current/constant voltage) is where you stay at the absorb voltage until you run out of sun.
Charging Voltages: Killing Batteries with Kindness
When I first got my battery bank, I set my charge voltages per the T-105 RE data sheet from the website when I got the pack. In a nutshell, absorb to 2.35-2.45V/cell, float at 2.20V/cell, equalize at 2.58V/cell. Or, for my pack, 56.4V-58.8V for absorb, equalize at 61.92V, float at 52.8V.
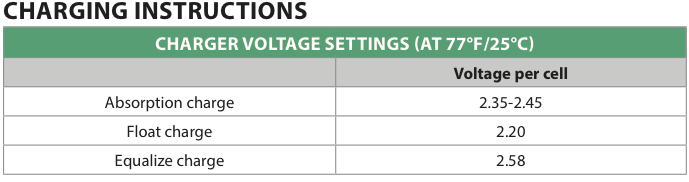
What are these voltages? A set of compromises, really, between perpetual undercharging and overcharging.
A year or so later, I went looking for the datasheet again to verify something, and discovered that the voltages had changed! The new recommendations: Absorb to 2.47V/cell, float at 2.25V/cell, equalize at 2.7V/cell. Or, for my pack, absorb at 59.28V, float at 54.0V, equalize at a whopping 64.8V. That’s a rather significant bump up from the old suggestions, so I sent a query and asked about it. A tech support rep informed me that the new voltages were correct for all the T-105 REs, so I adjusted my settings and went on my way. It took a bunch more research to figure out why they’d changed the voltages - batteries were dying early from sulfation or stratification in off-grid use, and the increased voltages improve the changes that the batteries will be fully charged and not die an early death from chronic undercharging.
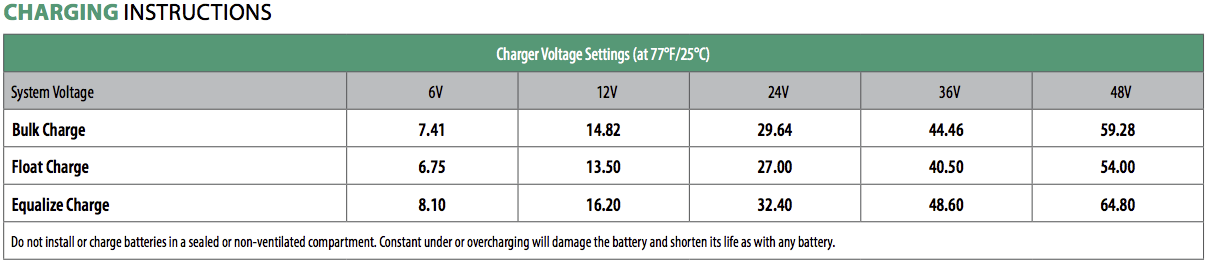
The older datasheets had voltage ranges - and most people, if they don’t understand lead acid, will likely pick something on the lower end of the range to be “nicer” to the cells. I did, not knowing any better (I think I used 2.40V/cell - smack in the middle of a range is good, right?). I knew my way around lithium, and lower charging voltages are easier on the cells. Not so for lead, if they’re not getting fully charged. Lower charging voltages on lead are quite literally “killing them with kindness.” Trojan’s revised datasheets eliminate the ambiguity and tell you to beat on them, hard.
This parallels nicely with some other reading I was doing at the time, talking about the charging method called…
“Max Smoke” Charging
Back in 2015, SunKing wrote a post on SolarPanelTalk entitled, “Are You Killing Your Batteries?” He talks about the inadequate charging from solar, and how a lot of battery manufacturers are seeing heavy warranty claims from renewable energy service batteries that sulfate up badly well inside the warranty period - and proposes that the solution is to basically treat them like you hate them, and charge them hard. Check the specific gravity in the evening, and adjust as needed. If they’re not fully charged, increase the voltage. If that doesn’t work, well, you might not have enough panel, so buy more solar panel or run your generator.
He also suggests that there is no point in doing three stage charging (bulk, absorb, float) off grid - there aren’t enough hours in the day to go through that cycle, so just skip the float state and try to cram as much charge into the cells as you can before the sun goes down. For most systems, especially a somewhat underpaneled system, this is really good advice. It doesn’t quite hold if you have a ton of panel (like I do). My overnight loads also are fairly low as I’m not working out there. My system is often into absorb by 10AM on a sunny morning, so I can get my batteries fully charged and into float on a typical day. It really depends on my energy use and the day’s solar conditions, but I very much can get my batteries fully charged on a sunny day.
One of the reasons my system works so well (in terms of overnight recovery) is that I have east facing “morning panels” on my office. These do a great job of soaking up the morning sun and putting it in my bank. Because they’re producing nearly full power when the sun is still glancing across my main array, I get an extra hour or two of charging time in - every single day.
If you don’t want to go outside regularly to check your specific gravity, you can also terminate your absorb charge stage with the current.
Current Based Charge Termination
Deciding when to drop out of absorb (if you have enough panel) is normally configured as a timed process, but this isn’t really the best way to do it (it’s better than nothing, only barely). A far better way is to monitor the current going in or out of the battery bank. During the absorb stage, when the current drops to 1-3% of the C/20 capacity (check your datasheet), the battery bank is full and you can safely switch back to the float voltage (if there’s still sun left). This works a bit closer to specific gravity based termination, and it will handle heavily drained batteries properly (they’ll sit at absorb voltages for a long while, and may not even finish the first day of charging).
This requires a nice shunt as the first item off the negative battery pack terminal and a way to get the data into the charge controller. Feed the actual battery charging current into your charge controller (the Midnite Classic WhizBang Jr does this nicely), and you can set up current based charge termination. It’s not perfect, but it does (hopefully) prevent continuing to gas fully charged batteries.

Normally, you’ll set this up, and also have a timer in the process as well - so the shunt can terminate charging early, but not extend the absorb time indefinitely. I’ve chosen to set my termination current low - it’s at 1% of my C/20 capacity, and I regularly hit that on sunny days, but it’s more charging than I might actually need. Since I do a lot of partial state of charge operation in the winter, I’d rather cram more in. I’ll likely adjust this up in the summer when I’m full every day.
When you get this running, you’ll notice that the absorb time varies, wildly. Coming off a late spring day with good sun, it might only take 2 hours to finish the absorb stage. In the winter, after a week of low batteries, it might take two full days of charging to get the current down far enough to enter float.
Net Charge Monitoring
Another really nice feature of the Whiz Bang Jr (and other similar units) is that they allow you to see the net charge on the battery - it allows counting amp-hours in and amp-hours out to get an idea as to the battery state of charge. I really, really like this little gizmo.
What this means, in practice, is that my charge controller knows something useful about my battery bank and can estimate capacity remaining. I set the rated amp-hour capacity, a charge efficiency percentage, and the temperature to capacity curve (sort of). The controller monitors the current and the battery pack temperature. This lets the charge controller estimate the current capacity of the pack (in amp-hours and percent), and track state of charge somewhat usefully. It’s not perfect, because it doesn’t appear to take the Peukert exponent into effect (yet another quirk of lead acid batteries where the faster you discharge it, the fewer amp-hours you get out), but it’s a solid first order approximation of the state of charge, and gets you into the ballpark. Plus, this allows you to easily see if you’re charging or discharging under a particular load. Usually, during the day, I try to make sure I’m charging, at least somewhat (which is not the case here - I’m drawing 4.2A out of the battery pack).

If you install the shunt when you get your battery pack, it will let you track lifetime amp hours in and out. I haven’t had this in for more than a few months, but you can see that I’ve shoved about 8.7% more amp-hours than I’ve taken out. This is consistent with operating on the overcharge side of lead acid, and is enough that I should be keeping my electrolyte pretty well stirred.

Watering
Just like your garden, flooded batteries need water. Distilled water only, please. Tap water and well water have enough extra stuff dissolved in them that they’ll destroy a battery in short order. If you don’t have your own water distiller, just get a few gallons at your local grocery store. It works fine.
Only water your batteries when they’re fully charged! The electrolyte volume increases as the battery charges, so if you top them off when drained, there’s a good chance you’ll have an acidic mess all over your battery box when charged. That’s just no fun, so don’t do that.
For watering, there are a few methods you can use. For all of them, the goal is to get the water to within about 1/8” of the bottom of the fill wells. Dumping water in with a gallon jug works, but it’s rather imprecise and amazingly messy (but a good way to clean the top of your battery bank).
You can buy watering systems, and if your batteries live inside where it doesn’t freeze, this is a really good option. Stick a hose in a gallon jug, pump until the batteries are all full, and you’re done. Easy, consistent, and will water a really big bank without much effort.
For those who have batteries outside (or really like checking specific gravity regularly), the watering systems are a bit of a problem. It’s hard to get the water out of them before it freezes, and the caps aren’t particularly easy to remove for checking SG. So, you should use a battery watering jug!
What’s a battery watering jug? A gizmo designed specifically for watering flooded lead acid batteries. There’s a sealed container, and a two stage nozzle that waters the batteries to the proper level. It’s a really neat little invention, and I highly suggest picking one up from eBay if you have flooded batteries!

The watering jug lets water flow until the level hits a certain point, at which time the airflow is blocked and the water stops flowing. You press it down into the fill well, and can feel it burbling. When it stops, the cell should be full!
Pay attention to the time it takes as well - sometimes mine won’t start flowing properly, and I’ll have to let it off and press down again. You’ll figure it out pretty quickly. These are amazing devices for watering batteries, and make it a ton easier to water the battery pack more regularly.
I like to toss a bit of an equalize charge on my pack after watering (half an hour or so), but that’s not required. It just helps mix up the electrolyte a bit from the aggressive gassing.
When you water the pack, you may notice that under load voltages seem to be down a bit after a charge (it’s most noticeable in the morning after a night of running loads). This is expected. When the battery is low on water, the specific gravity is higher (the same amount of acid, but less water). This leads to a higher voltage. When you water the battery, you bring the SG back into spec, and this will typically lower the voltage. I’ll offer that if you notice this effect strongly, you’ve waited far, far too long to water your batteries. When in doubt, water an off grid system every 3 months or so. You can water it more frequently, and some systems may need that, but every 3 months seems to work for mine nicely (I can go longer without exposing the plates, but I’ll see more of a voltage change and will require a lot of water).
Magic Powders and Fizzy Pills and Other Nonsense
You may be able to guess, from the section title, what I’m going to say about various battery additives and such. You shouldn’t use them. If you have a totally beat up bank and want another few months out of it, some of them might work. For a bit. At the cost of pretty much destroying the remainder of the battery in the process. They have no business in an off grid power system, and if you’re properly maintaining your battery bank, there’s no reason to even consider them. Run the batteries properly, and when they eventually get old and stop having enough useful capacity (or short a cell or something), replace the bank. Simple. Almost all the failures of lead acid are not easily reversed, and those that are reversible (sulfation) shouldn’t happen if you run the bank properly. So, treat them as snake oil and keep them appropriately far away from your bank.
Of the various failures, you may be able to reverse some sulfation. Anything that goes into the electrolyte is unlikely to help you long term (you may reverse some sulfation in exchange for destroying the remainder of the plates with corrosion). If any of the magic liquids and fizzy tablets were any good, battery manufacturers would include them in the battery to start with or offer them as extended life additives to be put in years later.
Lead-Carbon and Partial State of Charge Cycling
Finally, I want to finish by talking about some of the newer developments in lead acid batteries and how they can impact operations. You’ll see plenty of scattered hype about lead acid batteries and new developments. Some smell suspiciously of marketing snake oil (“lead silicone” or “silicone crystal” come to mind here - they’re some marketing applied to gel lead acid batteries, and you can find my opinion on those at the top of this post), but some are genuinely exciting. The various “lead carbon” developments fall into the second category.
You’ll see them under assorted names. Trojan calls it “Smart Carbon,” but most of the battery manufacturers have something along these lines in at least some of their batteries. You’re a lot more likely to find it in a renewable energy line than in a floor sweeper or forklift line of batteries, because the benefits in something charged from the grid every night are less substantial.
What these various technologies refer to is the addition of carbon to the negative electrode of a lead acid battery. Somehow, this seems to reduce the severity of sulfation buildup on the plate. It makes the crystals smaller so they’re easier to dissolve, or it keeps them from hardening, or… something like that. The end result is a lead acid battery that doesn’t sulfate the plates up as badly during sustained partial state of charge operation, and the sulfation dissolves easier when you try to recharge it. I can’t find many details on the particular nature of the operation, which probably means that nobody actually knows exactly how it works (or if they do, they don’t care to share). Just that it does. Battery research is weird like that. Read enough, and you realize there’s an awful lot of speculation around how things work, even if it’s known they work quite well.
This is very, very exciting for off grid energy use!
It means that instead of lead acid requiring regular full charges, you can get around to charging it fully a bit less frequently. You’ll still need to do that every so often, and regular deep discharges still hurt cycle life significantly, but during the winter, when power is scarce, there’s no reason to run the generator long enough to get the batteries through a full absorb cycle on a regular basis. Just wait for the sun to come out.
I’ve been doing this with my pack this past winter (my T-105 RE batteries have “Smart Carbon” which claim to be this variety of magic). If I have sun, the pack charges fully. If I’m dealing with inversions, I’ll run the pack down to about 60%, then run the generator up to about 85% (which is when I run the voltage up enough to hit the absorb voltage on a cold pack). And then turn the generator off. Since, in theory, my pack can handle longer periods of partial state of charge, I’ll keep it in the efficient operating range while I’m on generator, and then top it off with the sun. I’ve noticed that after doing this for a week, a full day of sun won’t drop my charge current down to the 1% mark I use for the winter endpoint, which means the batteries are still charging even on the second day.
But it beats sitting there with my generator idling along, burning fuel, trying to top off batteries that just don’t need to be topped off. I went through about half the generator fuel this winter as I did last winter when I didn’t understand my battery bank as well.
Lead Acid/Lithium/Nickel Iron
I expect this post will get some of the usual astonishment that I use lead acid batteries, when lithium is so clearly superior for everything (usually followed by naming some particularly well known name of lithium storage battery that isn’t usefully for sale unless you want to preorder and “be on the list” for an indefinite period of time, and then hope someone calls you, eventually). I’m quite aware of lithium - I do an awful lot of lithium battery rebuilding (I’ve rebuilt and sold quite a few kWh of lithium battery packs). But, at the same time, my office (where I rebuild lithium batteries) is powered by lead acid, and I see no reason to change that any time soon. It’s cheap enough, it works well enough, and since I’m heavily overpaneled for my loads, I can keep them fully enough charged, enough of the year, that I shouldn’t have any problems (yes, that was one of the more handwavy sentences I’ve written in this blog). Plus, they can sit outside and I don’t have to heat or cool them. They work fine for stationary storage, and the carbon additives to the negative electrode only help improve their suitability for that.
So, yes, there are places for lithium. Just not most off grid power systems. I’ll touch on this more in some future posts. If you’re serious about off grid lithium, look at LiFePO4.
I also regularly get questions about nickel iron (“Edison”) batteries. Go read up on people who actually have systems. They guzzle distilled water, they’re as loud as a den of snakes when charging, and are prone to carbonate poisoning from the atmospheric carbon they suck up. Neat idea, just not as practical as the glossy brochures claim.
Final Thoughts
This is a lot of information on lead acid batteries. It’s the result of over a year of research and reading on my part, trying to better understand how lead acid works, how lead acid fails, and how to keep lead acid alive. And there’s no guarantees here - sometimes you just roll 1s and a cell or two shorts out on you a few years in. But, understanding the failure mechanisms, and understanding how to mitigate them, should help an awful lot with longevity.
Have enough panel to charge them regularly, keep them watered, and check the specific gravity every now and then. That’s the core of it.
I’m sure I’ve missed some point or another, and I’d love feedback in the comments. However, if you’re going to go on about how some magical fizzy pill is going to “restore old batteries to brand new,” it’s unlikely your comments will stick around long. And, please, remember that this is all in the context of off grid lead acid systems. Keep it on topic. Thanks!
Comments
Comments are handled on my Discourse forum - you'll need to create an account there to post comments.If you've found this post useful, insightful, or informative, why not support me on Ko-fi? And if you'd like to be notified of new posts (I post every two weeks), you can follow my blog via email! Of course, if you like RSS, I support that too.